List of technologies developed by ICAR-National Research Centre onEquines, Hisar and available for transfer and commercialization
Equine Vaccines
- Equiherpabort vaccine.
- Updated Equine Influenza Vaccine.
Equine Disease Diagnostic kits/assays
- Equiherpes B-ELISA Kit.
- Recombinant gG-based type-specific ELISA for differentiation of EHV1 4 Infection.
- Recombinant gG-based type-specific ELISA for differentiation of EHV1 4 Infection.
- Equip Rotavirus kit.
- EIA ELISA kit
- Glanders ELISA Kit
- A highly sensitive kit for detection of antibodies against Theileria equi in serum of equids.
- Lateral flow assay for diagnosis of equine piroplasmosis.
Equine Reproduction Technologies
- A pregnancy diagnostic kit for equine based on detection of eCG by ELISA (Pregmare Kit).
- Semen cryopreservation and artificial insemination in equines.
Equine Vaccines
Inactivated vaccine for control of herpesvirus-1 (EHV-1) infection in horses
- Name of Technology: Equiherpabort vaccine
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
Name: Dr. T.K. Bhattacharya
Title: Director, NRCE
Telephone: +91-1662-275787, 276748, 282500,
Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details:
Equine herpes virus-1 (EHV-1) is responsible for heavy economic losses to the equine industry. Healthyhorses acquire infections mostly through respiratory tract. In a national assessment of EHV-1 infectionltamongst equidae in India, 13.5% serum samples were found seropositive. Killed vaccine isltrecommended for immunoprophylaxis in countries where there is a general ban on live vaccine. ICAR-ltNRCE has developed an inactivated vaccine developed for control of herpesvirus-1 infection in horses
Using field strain of EHV-1. This vaccine will serve as an alternative for control of the disease.ltEquiherpabort vaccine is a formalin inactivated vaccine prepared from an indigenous EHV-1 (strainlthisar-90-7), grown in VERO Cell culture. This is an oil emulsion mannide monooleate (OEMM) EHV-lt1 vaccine.
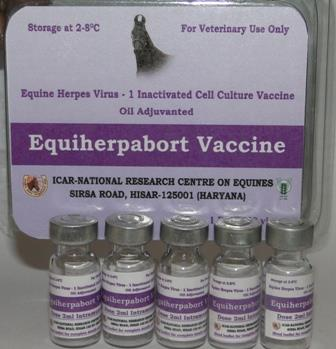
This vaccine is intended for all equine species especially for pregnant mares. Protectiveltimmunization response of this vaccine was recorded in experimental field trials conducted in pregnantltmares. This vaccine provides very good protective immunity against abortions due to EHV1. Thisltvaccine showed comparable results with respect to commercial vaccine. This vaccine will help inltdecrease in occurrence of abortions, paralysis, perinatal foal mortality and respiratory disease due toltEHV-1.
Licensing terms:
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
B. Updated Equine Influenza Vaccine against Equine Influenza virus (EIV)
- Name of Technology: Updated Equine Influenza Vaccine
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details:
Equine influenza – commonly known as ‘Horse Flu’ – is a viral disease of horses caused by EquineInfluenza virus (EIV) subtype H3N8. The disease resulted in heavy morbidity and led to huge economicloss. To control the disease, an inactivated aluminum hydroxide adjuvant vaccine was developed byNRCE consequent to 1987 outeak using Ludhiana/87 isolate which belonged to pre-divergent lineageof the EIV isolates. The EIV isolates were belonged to the Clade 2 of Florida sub lineage of Americanlineage on the basis of sequence analysis of haemagglutinin (HA) gene. Subsequently, the old vaccinewas updated by incorporating the EIV isolate – A/eq/Katra (Jammu)/06/08 (H3N8).

The vaccine wasmade from the seed stock of EIV grown in bulk in 9-11 days old emyonated chicken eggs and theallantoic fluid was harvested, purified by ultracentrifugation and inactivated by formalin. The vaccinewas tested as per the standard procedures laid down by OIE, European Pharmacopoeia and EuropeanMedicines Agency (EMEA) and showed protective immune response against EIV.
This vaccine isintended for immunization of horses, mules and donkeys. This vaccine will help in controlling influenzain equines during exigency and thus improves the economic status of equine owner and the state. It alsohelps in increase in equine production and their work efficiency. Vaccine is intended forimmunization of horses, mules and donkeys. First vaccination in animals above 6 months ofage followed by a booster vaccine after 4-5 weeks and repeated annually or after monitoringthe titres by Haemagglutination inhibition assay (HI titres below 64- repeat vaccine).
Licensing terms:
- Nature of License: Non-exclusive
- Duration of the Licensingense: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
Equine Disease Diagnostic kits/assays
A. monoclonal antibody-based blocking ELISA Kit for fast detection of equine herpesvirus-1(EHV-1) infection
- Name of Technology: Equiherpes B-ELISA Kit
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)

Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: Infection due to EHV-1 causes abortion, stillbirths, foal mortality, respiratory and neurological diseases in horses. EHV-1infection led abortion has been reported in ~15% pregnant mares in organized farms which incurs huge economic losses. Around 13.5% seropositivity was found in a national assessment of EHV-1 infection amongst equidae in the country. Recently, incidences of neurological form of the disease were also increasing globally. This ELISA kit was developed for diagnosis of equine herpesvirus-1(EHV-1) infection in horses. This kit is now in routine use at NRCE for sero-surveillance and disease investigation services. Equiherpes B-ELISA Kit is an alternative to virus neutralization test due to addition of neutralizing monoclonal antibody (mAb) in the assay. This kit gives results within 6 hours. This kit is able to detect seroconversion. Equiherpes B-ELISA Kit is useful for assessment of herd immunity in equine breeding farms where vaccination is undertaken against EHV-1. This kit has been validated internally/externally.This kit will help in timely diagnosis of EHV-1 infection and useful for assessment of herd immunity in equine breeding farms where vaccination is undertaken against EHV-1.
6. Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
B. A recombinant gG-based type-specific ELISA for differentiation of EHV1 4 Infection
- Name of Technology: EHV1/4 ELISA Kit
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: Equine herpesvirus-1 (EHV1) and equine herpesvirus-4 (EHV4) together responsible for ‘Equine Rhinopneumonitis’, an OIE listed disease of equines. EHV-1 in addition is foremost cause of abortions, neurological disorders and perinatal foal mortality. Disease is endemic across the globe including India. The differential diagnosis of EHV-1 and EHV-4 viruses is often complicated due to antigenic cross-reactivity between the two viruses. A recombinant glycoprotein G based ELISA for differentiation and

diagnosis of EHV1 and EHV4 was developed by NRCE and the kit is being routinely use in sero-surveilance at the Centre. The kit developed is a recombinant protein-based ELISA kit for differentiation and diagnosis of EHV1 and EHV4 infection. The kit has capacity to test 32 samples which includes three controls. The kit is validated internally and externally using field serum samples. The test is useful for assessment of herd immunity in equine breeding farms where vaccination is undertaken against EHV-1. Thus, this kit will help in timely diagnosis, differentiation and control of EHV1 and EHV4 infection. The ELISAV1 4 kit will prove beneficial to bio-pharmaceutical companies, state animal husbandry departments and small entrepreneurs.
Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
C. A monoclonal antibody (mAb)-based sandwich ELISA for detection of Rotavirus1. Name of Technology: Equip Rotavirus Kit2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: Rotaviruses are the major etiologic agents of severe, acute dehydrating diarrhea resulting in high morbidity and mortality in neonatal animals (including foals) and human infants. A monoclonal antibody (mAb)-based sandwich enzyme immunoassay has been developed for diagnosis of rotavirus in different animals and human stool samples. This kit employs a polyclonal anti-rotavirus serum as coating antibody to capture the rotavirus antigen and a monoclonal antibody is used for detection of group-specific antigen present on the captured rotaviruses. The mAb raised against group-specific protein VP6 of rotavirus, for detection of mammalian group A rotaviruses. This kit employs a polyclonal anti-rotavirus serum to capture the rotavirus antigen and a monoclonal antibody detect group-specific antigen present on the captured rotaviruses. The assay has 100% sensitivity and a specificity of 96% in comparison to virus isolation. This kit will help in timely diagnosis of rotavirus associated diarrhoea in animal and human population.
Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
D. An ELISA kit for detection of Equine infectious anaemia (EIA)
- Name of Technology: EIA ELISA kit
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
. Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: Equine infectious anaemia (EIA) – a chronic and debilitating retroviral disease of equids. The diagnosis of the disease is mainly based on identification of inapparent carriers by detection of antibodies against EIA virus (EIAV). EIA diagnosis is routinely done with imported Agar Gel Immunodiffusion (AGID) kit. It generally takes 18-24 hrs (sometimes 48 h) for interpretation of results.
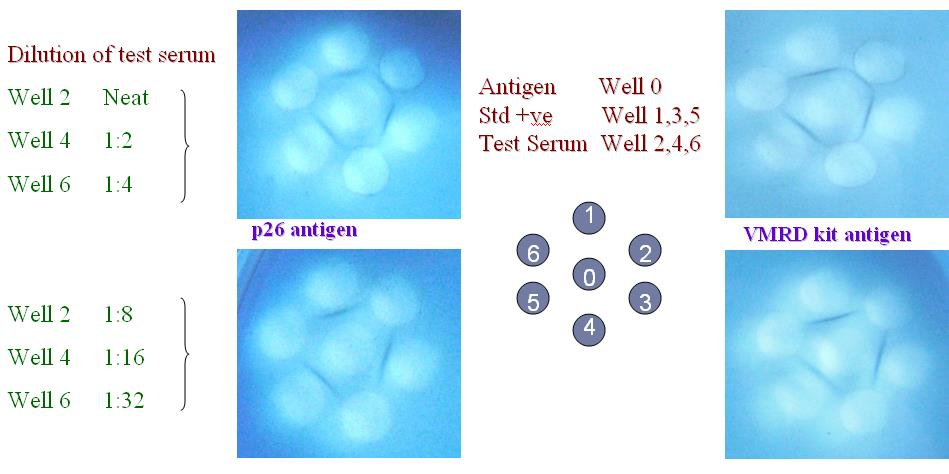
It also requires expertise in the field for the subjective interpretation of the result and cost for testing sample with the existing kit is also higher. ICAR-NRCE has developed a recombinant antigen (p26) based indirect ELISA kit for diagnosis of equine infectious anaemia. The test detect antibody specific to EIA virus. This kit is faster than AGID and it takes 3-4 hrs to complete the assay and result interpretation. This kit has high relative sensitivity (100%) and specificity (98.7%) in comparison with AGID assay. EIA-ELISA kit has test capacity of 45 samples per plate and stability of kit reagents is one year at 4 o C. This kit is cost-effective as compared to imported kit and helpful in eradicating/declaring EIA-free nation which will be invaluable in terms of exporting our equines in EIA free country.
Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
E. A Recombinant antigen based ELISA Kit for diagnosis of Glanders
- Name of Technology: Glanders ELISA Kit
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: Glanders is a fatal bacterial disease of equines caused by non-motile gram-negative bacterium Burkholderia mallei. Because of zoonotic importance of the glanders and frequent occurrence of the disease in parts of Africa, the Middle East, South America, and Asia, glanders has attracted renewed research interest and is increasingly recognized worldwide as potential biological weapons. In India,glanders cases have been reported almost in every year since 2006. The Complement Fixation test (CFT) is an OIE prescribed sero-diagnostic method for glanders for international trade of equidae. The analytical performance of the test was found to be heavily depends on CFT antigens used from different commercial source and differences in the recommended protocol. False-negative glanders test results may lead to the introduction of the infectious agent into a glanders free-region and false-positive test results may lead to unnecessary restrictions on international trade of animals leading to financial losses for owners and the equine industry. In order to improve test quality, three recombinant proteins (Hcp1, TssA and TssB) based indirect ELISAs have been developed by NRCE for detection of B. mallei specific antibodies in equines. These kits were evaluated with large number (n= 2000 to 15000) of equine serum samples obtained from glanders free as well as glanders endemic areas. The diagnostic specificity of the assays were found to be 90-100% and sensitivity of the three assays ranges from 90- 99%, respectively.
Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
F. A Recombinant antigen based ELISA kit for diagnosis of equine Piroplasmosis (Theileria equi)
- Name of Technology: Theileria equi ELISA kit
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: Equine piroplasmosis – also called equine babesiosis – is an acute, sub-acute or chronic tick-borne disease of equids, caused by an intra-erythrocytic protozoa, Theileria equi (T. equi) or Babesia caballi. Recently, NRCE has developed recombinant erythrocyte merozoite antigen-2 (EMA-2) – based ELISA for detection of antibodies against T. equi parasite in equine serum. This ELISA in being used routinely for detection of antibodies against Theileria equi parasite in equine serum at NRCE.

The kit has ready-to-use ELISA plate onto which serum samples can be loaded after proper dilution. The test sensitivity and specificity is comparable to OIE recommended ELISA. This kit is highly sensitive and helps in early detection of disease along with r-ELISA gave uniform results and no batch to batch antigen variation is observed.
Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
G. A rapid assay for detection of antibodies against Theileria equi infection in equines
- Name of Technology: Lateral flow assay for detection of antibodies against Theileria equiinfection in equines
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: Equine piroplasmosis is an acute, sub-acute or chronic tick-borne disease of equines, caused by an intra- erythrocytic haemo-protozoa Theileria equi or Babesia caballi. Significant segment of the Indian equine population (~35%) is latently infected and diagnosis of these animals is of more
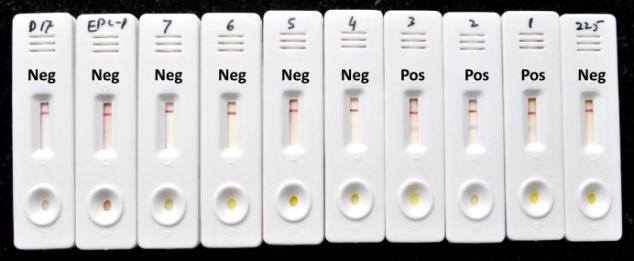
relevance to prevent spread of the parasitic infection to naïve animals. In an effort to provide a farmer friendly field test kit, the Centre has successfully developed lateral flow assay (LFA) for diagnosis of T. equi infection. The kit is based on a recombinant T. equi merozoite surface antigen (EMA-2) conjugated with gold-nano particles. The diagnostic sensitivity (Dsn) and specificity (Dsp) of LFA vis-à-vis ELISA were 0.945 and 0.916, respectively, indicating its applicability on the field samples. LFA has many advantages as compared to ELISA, such as no equipment or trained personnel needed and visually readable results obtained within 10 minutes.
Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interestedbuyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
Equine Reproduction Technologies
A. An early pregnancy detection ELISA kit for equine
- Name of Technology: Pregmare Kit
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: This is an early pregnancy kit based on ELISA which can be used for pregnancy diagnosis between 35 to 120 days of gestation in mares covered by horse stallions. It is based on the detection of PMSG or eCG which is released after conception (after about 30 days of gestation) from the endometrial cups in pregnant mares. This kit is now in routine use at for pregnancy diagnosis in mares at field level. This can be used both for early pregnancy and foetus viability. This kit can be used both as qualitative and quantitative test. This ELISA kit is rapid, sensitive, specific and animal friendly. Kit is stable for at least 6 months at 4 o C. Economically, it is very beneficial to all categories of equine owners as cost per test is quite low as compared to ultrasound scanning fee.

Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
B. Semen cryopreservation and artificial insemination in equines
- Name of Technology: Semen cryopreservation and artificial insemination
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details: Due to non-availability of good quality stallion and Jacks with the equine owners in the country for producing good quality horses, donkeys and mules, artificial insemination is can be used for the genetic improvement of equines. Cryopreservation and Artificial insemination techniques are mainly used for breed improvement as well as for controlling of venereal diseases in equids. These techniques are safe and more number of foals can be produced in a year by judicious use semen of the valuable stallion. Above techniques will ensure the availability of good quality semen and genetic improvement of the equines. This technique is safe and more number of foals can be produced in a year by semen of judicious use of the valuable even a single good quality stallion. Besides, cryopreserved semen can be transported over a long distance without any difficulty hence this technique is useful for small and marginal farmers as well as for organized farms owners, it can be adopted at all levels. This technique will prove helpful in improving overall socio-economic status and the conditions of the equine owners as the cost of superior mule/true-to breed equids is high. These techniques are useful for up-gradation of donkeys/horses as well as for superior quality mule production.
Licensing terms
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer/organisation.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
Equine Vaccines
Inactivated vaccine for control of herpesvirus-1 (EHV-1) infection in horses
- Name of Technology: Equiherpabort vaccine
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
5. Technology Details:
Equine herpes virus-1 (EHV-1) is responsible for heavy economic losses to the equine industry. Healthyhorses acquire infections mostly through respiratory tract. In a national assessment of EHV-1 infectionltamongst equidae in India, 13.5% serum samples were found seropositive. Killed vaccine isltrecommended for immunoprophylaxis in countries where there is a general ban on live vaccine. ICAR-ltNRCE has developed an inactivated vaccine developed for control of herpesvirus-1 infection in horses
using field strain of EHV-1. This vaccine will serve as an alternative for control of the disease.ltEquiherpabort vaccine is a formalin inactivated vaccine prepared from an indigenous EHV-1 (strainlthisar-90-7), grown in VERO Cell culture. This is an oil emulsion mannide monooleate (OEMM) EHV-lt1 vaccine. This vaccine is intended for all equine species especially for pregnant mares. Protectiveltimmunization response of this vaccine was recorded in experimental field trials conducted in pregnantltmares. This vaccine provides very good protective immunity against abortions due to EHV1. Thisltvaccine showed comparable results with respect to commercial vaccine. This vaccine will help inltdecrease in occurrence of abortions, paralysis, perinatal foal mortality and respiratory disease due toltEHV-1.

6. Licensing terms:
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
Updated Equine Influenza Vaccine against Equine Influenza virus (EIV)
- Name of Technology: Updated Equine Influenza Vaccine
- Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)
- Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information
Contact details:
- Name: Dr. T.K. Bhattacharya
- Title: Director, NRCE
- Telephone: +91-1662-275787, 276748, 282500
- Email: nrcequine@nic.in
Section 2: Technical Description
Technology Details:
Equine influenza – commonly known as ‘Horse Flu’ – is a viral disease of horses caused by EquineInfluenza virus (EIV) subtype H3N8. The disease resulted in heavy morbidity and led to huge economicloss. To control the disease, an inactivated aluminum hydroxide adjuvant vaccine was developed byNRCE consequent to 1987 outeak using Ludhiana/87 isolate which belonged to pre-divergent lineageof the EIV isolates. The EIV isolates were belonged to the Clade 2 of Florida sub lineage of Americanlineage on the basis of sequence analysis of haemagglutinin (HA) gene. Subsequently, the old vaccinewas updated by incorporating the EIV isolate – A/eq/Katra (Jammu)/06/08 (H3N8). The vaccine wasmade from the seed stock of EIV grown in bulk in 9-11 days old emyonated chicken eggs and theallantoic fluid was harvested, purified by ultracentrifugation and inactivated by formalin. The vaccinewas tested as per the standard procedures laid down by OIE, European Pharmacopoeia and EuropeanMedicines Agency (EMEA) and showed protective immune response against EIV.
This vaccine isintended for immunization of horses, mules and donkeys. This vaccine will help in controlling influenzain equines during exigency and thus improves the economic status of equine owner and the state. It alsohelps in increase in equine production and their work efficiency. Vaccine is intended forimmunization of horses, mules and donkeys. First vaccination in animals above 6 months ofage followed by a booster vaccine after 4-5 weeks and repeated annually or after monitoringthe titres by Haemagglutination inhibition assay (HI titres below 64- repeat vaccine).

Licensing terms:
- Nature of License: Non-exclusive
- Duration of the License: Will be decided after mutual discussion with the interested buyer.
- Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.
- Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.
- Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
- Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.
Section 3: Certifications and Approvals
It is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
Equine Disease Diagnostic kits/assays
A. monoclonal antibody-based blocking ELISA Kit for fast detection of equine herpesvirus-1(EHV-1) infection
1. Name of Technology: Equiherpes B-ELISA Kit2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)
Section 1: Contact Information Contact details:• Name: Dr. T.K. Bhattacharya• Title: Director, NRCE• Telephone: +91-1662-275787, 276748, 276151, 275114 Email: nrcequine@nic.in
Section 2: Technical Description Technology Details:Infection due to EHV-1 causes abortion, stillbirths, foal mortality, respiratory and neurological diseases in horses. EHV-1infection led abortion has been reported in ~15% pregnant mares in organized farms which incurs huge economic losses. Around 13.5% seropositivity was found in a national assessment of EHV-1 infection amongst equidae in the country. Recently, incidences of neurological form of the disease were also increasing globally. This ELISA kit was developed for diagnosis of equine herpesvirus-1(EHV-1) infection in horses. This kit is now in routine use at NRCE for sero-surveillance and disease investigation services. Equiherpes B-ELISA Kit is an alternative to virus neutralization test due to addition of neutralizing monoclonal antibody (mAb) in the assay. This kit gives results within 6 hours. This kit is able to detect seroconversion. Equiherpes B-ELISA Kit is useful for assessment of herd immunity in equine breeding farms where vaccination is undertaken against EHV-1. This kit has been validated internally/externally.This kit will help in timely diagnosis of EHV-1 infection and useful for assessment of herd immunity in equine breeding farms where vaccination is undertaken against EHV-1

Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.f. Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
B. A recombinant gG-based type-specific ELISA for differentiation of EHV1 4 Infection1. Name of Technology: EHV1/4 ELISA Kit2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)Section 1: Contact InformationContact details:• Name: Dr. T.K. Bhattacharya• Title: Director, NRCE• Telephone: +91-1662-275787, 276748, 276151, 275114Email: nrcequine@nic.inSection 2: Technical Description5. Technology Details:Equine herpesvirus-1 (EHV1) and equine herpesvirus-4 (EHV4) together responsible for ‘Equine Rhinopneumonitis’, an OIE listed disease of equines. EHV-1 in addition is foremost cause of abortions, neurological disorders and perinatal foal mortality. Disease is endemic across the globe including India. The differential diagnosis of EHV-1 and EHV-4 viruses is often complicated due to antigenic cross-reactivity between the two viruses. A recombinant glycoprotein G based ELISA for differentiation and diagnosis of EHV1 and EHV4 was developed by NRCE and the kit is being routinely use in sero-surveilance at the Centre. The kit developed is a recombinant protein-based ELISA kit for differentiation and diagnosis of EHV1 and EHV4 infection. The kit has capacity to test 32 samples which includes three controls. The kit is validated internally and externally using field serum samples. The test is useful for assessment of herd immunity in equine breeding farms where vaccination is undertaken against EHV-1. Thus, this kit will help in timely diagnosis, differentiation and control of EHV1 and EHV4 infection. The ELISAV1 4 kit will prove beneficial to bio-pharmaceutical companies, state animal husbandry departments and small entrepreneurs.

6. Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.f.Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
C. A monoclonal antibody (mAb)-based sandwich ELISA for detection of Rotavirus1. Name of Technology: Equip Rotavirus Kit2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)Section 1: Contact Information4. Contact details:• Name: Dr. T.K. Bhattacharya Title: Director, NRCE• Telephone: +91-1662-275787, 276748, 276151, 275114
Email: nrcequine@nic.inSection 2: Technical Description Technology Details:Rotaviruses are the major etiologic agents of severe, acute dehydrating diarrhea resulting in high morbidity and mortality in neonatal animals (including foals) and human infants. A monoclonal antibody (mAb)-based sandwich enzyme immunoassay has been developed for diagnosis of rotavirus in different animals and human stool samples. This kit employs a polyclonal anti-rotavirus serum as coating antibody to capture the rotavirus antigen and a monoclonal antibody is used for detection of group-specific antigen present on the captured rotaviruses. The mAb raised against group-specific protein VP6 of rotavirus, for detection of mammalian group A rotaviruses. This kit employs a polyclonal anti-rotavirus serum to capture the rotavirus antigen and a monoclonal antibody detect group-specific antigen present on the captured rotaviruses. The assay has 100% sensitivity and a specificity of 96% in comparison to virus isolation. This kit will help in timely diagnosis of rotavirus associated diarrhoea in animal and human population.Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.f. Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
D. An ELISA kit for detection of Equine infectious anaemia (EIA)1. Name of Technology: EIA ELISA kit2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)Section 1: Contact Information Contact details:• Name: Dr. T.K. Bhattacharya• Title: Director, NRCE• Telephone: +91-1662-275787, 276748, 276151, 275114 Email: nrcequine@nic.in
Section 2: Technical Description Technology Details:Equine infectious anaemia (EIA) – a chronic and debilitating retroviral disease of equids. The diagnosis of the disease is mainly based on identification of inapparent carriers by detection of antibodies against EIA virus (EIAV). EIA diagnosis is routinely done with imported Agar Gel Immunodiffusion (AGID) kit. It generally takes 18-24 hrs (sometimes 48 h) for interpretation of results. It also requires expertise in the field for the subjective interpretation of the result and cost for testing sample with the existing kit is also higher. ICAR-NRCE has developed a recombinant antigen (p26) based indirect ELISA kit for diagnosis of equine infectious anaemia. The test detect antibody specific to EIA virus. This kit is faster than AGID and it takes 3-4 hrs to complete the assay and result interpretation. This kit has high relative sensitivity (100%) and specificity (98.7%) in comparison with AGID assay. EIA-ELISA kit has test capacity of 45 samples per plate and stability of kit reagents is one year at 4 o C. This kit is cost-effective as compared to imported kit and helpful in eradicating/declaring EIA-free nation which will be invaluable in terms of exporting our equines in EIA free country.

Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.f. Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
E. A Recombinant antigen based ELISA Kit for diagnosis of Glanders1. Name of Technology: Glanders ELISA Kit2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)Section 1: Contact Information Contact details:Name: Dr. T.K. Bhattacharya Title: Director, NRCE Telephone: +91-1662-275787, 276748, 276151, 275114 Email: nrcequine@nic.in Section 2: Technical Description Technology Details:Glanders is a fatal bacterial disease of equines caused by non-motile gram-negative bacterium Burkholderia mallei. Because of zoonotic importance of the glanders and frequent occurrence of the disease in parts of Africa, the Middle East, South America, and Asia, glanders has attracted renewed research interest and is increasingly recognized worldwide as potential biological weapons. In India,glanders cases have been reported almost in every year since 2006. The Complement Fixation test (CFT) is an OIE prescribed sero-diagnostic method for glanders for international trade of equidae. The analytical performance of the test was found to be heavily depends on CFT antigens used from different commercial source and differences in the recommended protocol. False-negative glanders test results may lead to the introduction of the infectious agent into a glanders free-region and false-positive test results may lead to unnecessary restrictions on international trade of animals leading to financial losses for owners and the equine industry. In order to improve test quality, three recombinant proteins (Hcp1, TssA and TssB) based indirect ELISAs have been developed by NRCE for detection of B. mallei specific antibodies in equines. These kits were evaluated with large number (n= 2000 to 15000) of equine serum samples obtained from glanders free as well as glanders endemic areas. The diagnostic specificity of the assays were found to be 90-100% and sensitivity of the three assays ranges from 90- 99%, respectively. Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.
f. Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
F. A Recombinant antigen based ELISA kit for diagnosis of equine Piroplasmosis (Theileria equi )1. Name of Technology: Theileria equi ELISA kit2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)Section 1: Contact Information Contact details:• Name: Dr. T.K. Bhattacharya • Title: Director, NRCE• Telephone: +91-1662-275787, 276748, 276151, 275114 Email: nrcequine@nic.in• Section 2: Technical Description Technology Details:Equine piroplasmosis – also called equine babesiosis – is an acute, sub-acute or chronic tick-borne disease of equids, caused by an intra-erythrocytic protozoa, Theileria equi (T. equi) or Babesia caballi. Recently, NRCE has developed recombinant erythrocyte merozoite antigen-2 (EMA-2) – based ELISA for detection of antibodies against T. equi parasite in equine serum. This ELISA in being used routinely for detection of antibodies against Theileria equi parasite in equine serum at NRCE. The kit has ready-to-use ELISA plate onto which serum samples can be loaded after proper dilution. The test sensitivity and specificity is comparable to OIE recommended ELISA. This kit is highly sensitive and helps in early detection of disease along with r-ELISA gave uniform results and no batch to batch antigen variation is observed.

Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.f. Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
G. A rapid assay for detection of antibodies against Theileria equi infection in equines1. Name of Technology: Lateral flow assay for detection of antibodies against Theileria equiinfection in equines2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)Section 1: Contact Information Contact details:• Name: Dr. T.K. Bhattacharya • Title: Director, NRCE• Telephone: +91-1662-275787, 276748, 276151, 275114Email: nrcequine@nic.inSection 2: Technical Description Technology Details:Equine piroplasmosis is an acute, sub-acute or chronic tick-borne disease of equines, caused by an intra- erythrocytic haemo-protozoa Theileria equi or Babesia caballi. Significant segment of the Indian equine population (~35%) is latently infected and diagnosis of these animals is of more relevance to prevent spread of the parasitic infection to naïve animals. In an effort to provide a farmer friendly field test kit, the Centre has successfully developed lateral flow assay (LFA) for diagnosis of T. equi infection. The kit is based on a recombinant T. equi merozoite surface antigen (EMA-2) conjugated with gold-nano particles. The diagnostic sensitivity (Dsn) and specificity (Dsp) of LFA vis-à-vis ELISA were 0.945 and 0.916, respectively, indicating its applicability on the field samples. LFA has many advantages as compared to ELISA, such as no equipment or trained personnel needed and visually readable results obtained within 10 minutes.

6. Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interestedbuyer.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.f. Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
Equine Reproduction TechnologiesA. An early pregnancy detection ELISA kit for equine1. Name of Technology: Pregmare Kit2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)Section 1: Contact Information Contact details:• Name: Dr. T.K. Bhattacharya • Title: Director, NRCE• Telephone: +91-1662-275787, 276748, 276151, 275114Email: nrcequine@nic.inSection 2: Technical Description Technology Details:This is an early pregnancy kit based on ELISA which can be used for pregnancy diagnosis between 35 to 120 days of gestation in mares covered by horse stallions. It is based on the detection of PMSG or eCG which is released after conception (after about 30 days of gestation) from the endometrial cups in pregnant mares. This kit is now in routine use at for pregnancy diagnosis in mares at field level. This can be used both for early pregnancy and foetus viability. This kit can be used both as qualitative and quantitative test. This ELISA kit is rapid, sensitive, specific and animal friendly. Kit is stable for at least 6 months at 4 o C. Economically, it is very beneficial to all categories of equine owners as cost per test is quite low as compared to ultrasound scanning fee.

Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.f. Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
B. Semen cryopreservation and artificial insemination in equines1. Name of Technology: Semen cryopreservation and artificial insemination2. Name of the Institute: ICAR-National Research on Centre on Equines (NRCE)3. Ownership of the technology: Indian Council of Agricultural Research (ICAR)Section 1: Contact Information Contact details:• Name: Dr. T.K. Bhattacharya • Title: Director, NRCE• Telephone: +91-1662-275787, 276748, 276151, 275114• Email: nrcequine@nic.in Section 2: Technical Description Technology Details:Due to non-availability of good quality stallion and Jacks with the equine owners in the country for producing good quality horses, donkeys and mules, artificial insemination is can be used for the genetic improvement of equines. Cryopreservation and Artificial insemination techniques are mainly used for breed improvement as well as for controlling of venereal diseases in equids. These techniques are safe and more number of foals can be produced in a year by judicious use semen of the valuable stallion. Above techniques will ensure the availability of good quality semen and genetic improvement of the equines. This technique is safe and more number of foals can be produced in a year by semen of judicious use of the valuable even a single good quality stallion. Besides, cryopreserved semen can be transported over a long distance without any difficulty hence this technique is useful for small and marginal farmers as well as for organized farms owners, it can be adopted at all levels. This technique will prove helpful in improving overall socio-economic status and the conditions of the equine owners as the cost of superior mule/true-to breed equids is high. These techniques are useful for up-gradation of donkeys/horses as well as for superior quality mule production. Licensing termsa. Nature of License: Non-exclusiveb. Duration of the License: Will be decided after mutual discussion with the interested buyer.c. Licensee fee and royalty: Will be decided after mutual discussion with the interested buyer/organisation.d. Handholding and training support required: Up to 3 Persons nominated by the licensee shall be trained for the period up to 5 days.e. Cost for handholding and training: The cost of training to be imparted by the institute will be included in the licensing fee. The expenses for boarding and lodging and travel of the licensee personal shall be borne by the licensee.f. Any other specific requirements: All statutory compliances related to production, sales, transportation and storage to be fulfilled by the licensee.22Section 3: Certifications and ApprovalsIt is certified that the above information about the Technology Nominated for Transfer of Technology is correct and no Security Sensitive/ Confidential and Proprietary information has been provided.
